4 QMS requirements 
4.1 General requirements
(Requirements 1 to 24)
ISO 9001 requirements, pitfalls to avoid
The requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2) of the ISO 9001: 2015 standard in sub-clauses of articles 4 to 10 are shown in figure 4-1:

Figure 4-1. Requirements of the ISO 9001 standard
Productany outcome of a process or activity (see also ISO 9000, 3.4.2) requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2) are specified by the customeranyone who receives a product (see also ISO 9000, 3.3.5), by the company or by regulation. The requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2) of the ISO 9001 standard concern exclusively the quality management systemset of processes allowing the achievement of the quality objectives (see also ISO 9000, 3.2.3) and its processes:
- external and internal issues are determined
- requirements of all interested parties are identified
- the scope of the quality management system (QMS) is established and documented
- the processes necessary for the QMS are identified, the corresponding resources ensured, the owners appointed and the interactions determined
- top management demonstrates leadership (takes responsibility)
- the quality policy, objectives, resources, competence, roles and work environment are determined
- actions to address risks are taken (see annexes 04 and 05)
- each process is measured, monitored, objectives established, followed-up and analyzed
- actions to achieve continual process improvement are established and implemented
Pitfalls to avoid:
- going overboard on quality
- having all procedures written by the quality manager
- forgetting the specificities linked to the corporate culture
4.2 Top management commitment
(Requirements 25 to 120)
Purpose, policy, objectives, indicators, commitments
In a company everyone assumes their responsibilities, but responsibility for quality begins with top management because, as the Romanian proverb says:
When you sweep the stairs, you start at the top
Top management defines the purpose of the company and ensures the sustainability, quality policy and continual improvementpermanent process allowing the improvement of the global performance of the organization (see also ISO 9000, 3.2.13 and ISO 14 001, 3.2) of the QMSquality management system (see figure 4-2). Quality objectives are deployed in each department and indicators are put in place to measure processactivities which transform inputs into outputs (see also ISO 9000, 3.4.1) performance.

Figure 4-2. Deployment of quality objectives
When policy is ambitious, it stimulates and motivates all staff
To achieve this, top management commits to:
- communicate the importance of customer requirements and regulations
- develop and promote the quality policy (see figure 4-3), which:

- is adapted to:
- the purpose of the company
- the context of the company
- available resources
- contributes to:
- meet customer and regulatory requirements
- continually improve the effectiveness of the QMS
- provides the framework for defining quality objectives, which are:
- SMART:
- Specific
- Measurable
- Achievable (and ambitious)
- Realistic, consistent with policy (neither unachievable nor too easy to achieve)
- Temporal (planned over time) and
- transformed into numerical indicators linked to:
- customer
- process
- product
- SMART:
- is communicated, understood and applied at all levels by everyone
- is adapted to:
- conduct management reviews at defined intervals to verify the achievement of quality objectives (see annex 03)
- identify and address risks (threats and opportunities) that could influence:
- the conformity of products and services
- the ability to increase customer satisfaction
- ensure the availability of human and technical resources to achieve quality objectives
- determine and respect customer requirements. Explicit or implicit customer requirements are transformed into internal requirements
- plan the QMS with the aim of satisfying general requirements and quality objectives
- maintain the proper functioning of the QMS during the introduction of any modification (internal modifications, new projects, products, processes)
- define and communicate responsibilities and authorities at all levels
Responsibility cannot be shared. Robert Heinlein

Figure 4-3. The Develop policy process
Top management: group or persons in charge of the organizational control at the highest level
Achieving objectives means respecting commitments made in the quality policy
Some examples of objectives:
- growth in turnover
- customer satisfaction
- staff stabilization
- staff competence
- improvement of maintenance
Some examples of indicators:
- evolution of market shares in %
- new customers
- customer return rate
- absenteeism in %
- % of staff trained
- response time in minutes
- machine downtime in minutes
 Minute of relaxation. See the “Gold contract” joke.
Minute of relaxation. See the “Gold contract” joke.
Staff are made aware of the:
- importance of respecting the:
- policy
- procedures
- instructions
- QMS requirements
- impact on quality related to each workstation
- positive effects coming from each person’s performance
- role and responsibilities for meeting QMS requirements
4.3 Product realization
(Requirements 121 to 291)
Planning, customers, satisfy requirements process, communication, design, development, purchases, production
4.3.1 Planning and customers
The productany outcome of a process or activity (see also ISO 9000, 3.4.2) production processes are planned and developed in accordance with the general requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2) of the QMSquality management system.
For the operational production of the productany outcome of a process or activity (see also ISO 9000, 3.4.2), an appropriate documentation (quality plan, project plan, productany outcome of a process or activity (see also ISO 9000, 3.4.2) plan) defines the:
- quality objectives to be achieved
- customer requirements related to the product
- deadlines to respect
- costs not to be exceeded
- production process
- documents (work instructions, product sheets)
- necessary resources (budget)
- activities of:
- verification
- validation
- monitoring
- inspection
- test
- measurement of customer satisfaction (questionnaires)
- acceptance criteria and
- records required
Verification: periodic inspection survey of compliance of a process, product or material
Validation: confirmation that the application of a process, product, service or material allows expected results to be achieved
Inspection: actions of measuring, testing and examining a process, product or material to establish whether requirements are met
Quality plan: document specifying the methods, means, responsibilities and stages of activities related to quality, applied specifically to a product, project or process
The only measure of quality is customer satisfaction
A processactivities which transform inputs into outputs (see also ISO 9000, 3.4.1) determines customeranyone who receives a product (see also ISO 9000, 3.3.5), legal and productany outcome of a process or activity (see also ISO 9000, 3.4.2) requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2) (see figure 4-4).
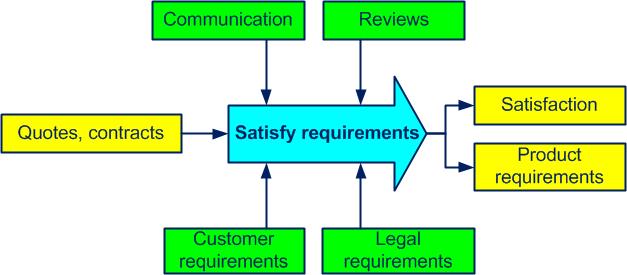
Figure 4-4. The Satisfy requirements process
Transparent communication arrangements with customers (see figure 4-5) are used regarding:
- information about the product (technical specifications, conditions of use and others)
- changes to the product or process
- consultations, contracts, amendments, waivers and orders
- customer information, especially complaints (delays, nonconformities, returns)
- actions put in place to eliminate nonconformities
Good news walks, bad news runs. Swedish proverb

Figure 4-5. Communicate process
 Minute of relaxation. See the “Lack of communication” joke.
Minute of relaxation. See the “Lack of communication” joke.
4.3.2 Design and development
I did not fail. I just found 10,000 ways that do not work. Thomas Edison
Each stage of the design and development processactivities which transform inputs into outputs (see also ISO 9000, 3.4.1) (we are not talking about research) is planned and controlled (see figure 4-6).
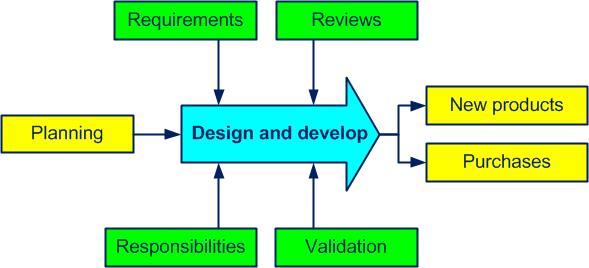
Figure 4-6. The Design and develop process
Responsibilities and authorities are determined, and effective communication between all personnel involved is ensured.
The requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2) for productany outcome of a process or activity (see also ISO 9000, 3.4.2) design and development inputs are:
- determined and recorded
- complete, unambiguous and non-contradictory
- reviewed regarding their adequacy
Inputs include data related to:
- functional requirements
- market needs
- customer expectations
- regulatory and legal requirements
- similar designs information
- other requirements
Before their use, the outputs of productany outcome of a process or activity (see also ISO 9000, 3.4.2) design and development are:
- verified and
- approved
In addition, they are adequate for the inputs and provide the information necessary to:
- purchases
- the production
- product acceptance criteria or a reference thereto
- the functional characteristics of the product
- safe use of the product
During the design and development processactivities which transform inputs into outputs (see also ISO 9000, 3.4.1), reviews are planned, recorded and carried out at key stages.
In this way:
- the ability to meet the requirements is assessed and
- problems are identified and solutions proposed
A problem well stated is a problem half solved. Charles Kettering
Verification (technical aspect), validation (functional aspect) and approval of design and development and their modifications are a logical continuation of the reviews; their records are preserved.
Any modification goes through a thorough review (technical and functional consequences) before being verified, validated and approved by the competent person(s).
4.3.3 Purchases and production
If you buy quality, you only cry once. English proverb
A processactivities which transform inputs into outputs (see also ISO 9000, 3.4.1) ensures that productsany outcome of a process or activity (see also ISO 9000, 3.4.2) purchased and services provided by external providers comply with requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2) at each delivery.
External providers are evaluated and selected according to defined criteria (compliant productany outcome of a process or activity (see also ISO 9000, 3.4.2), compliance with cost and deadlines). A reassessment is carried out periodically. Records of assessments, selections, reassessments and related actions are retained.
Purchasing information is used to describe the purchased productany outcome of a process or activity (see also ISO 9000, 3.4.2) and define requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2):
- for approval of:
- products
- procedures
- processes and
- equipment
- for staff qualification
- relating to the QMS
The implementation of services linked to the production of the productany outcome of a process or activity (see also ISO 9000, 3.4.2) and preparation of the service is validated in accordance with the specified requirementsexplicit or implicit need or expectation (see also ISO 9000, 3.1.2). Production (see figure 4-7) is carried out under controlled conditions leading to reproducible results.

Figure 4-7. The Produce process
These conditions may include means related to:
- product and process characteristics
- acceptance criteria
- work instructions
- appropriate equipment
- operator skills
- monitoring and measurement equipment and activities
- records required
- release, delivery and post-delivery service activities
When appropriate (almost always) the productany outcome of a process or activity (see also ISO 9000, 3.4.2) and its condition, in relation to the inspections carried out, are identified, recorded and the traceability of the productany outcome of a process or activity (see also ISO 9000, 3.4.2) is controlled throughout its production.
Traceability: aptitude to memorize or restore all or part of a trace of executed functions
Special care is taken for the customeranyone who receives a product (see also ISO 9000, 3.3.5)’s property (which may be intellectual property). This property is identified, verified, protected and backed up. For any incident that occurs, the customeranyone who receives a product (see also ISO 9000, 3.3.5) is notified immediately and records are kept.
The company ensures the completeness of the productany outcome of a process or activity (see also ISO 9000, 3.4.2) and its components with appropriate operations:
- identification
- intermediate storage
- handling
- packaging
- storage
- protection and
- delivery
Productany outcome of a process or activity (see also ISO 9000, 3.4.2) conformity is ensured with processes using appropriate and valid monitoring and measuring equipment. Any measuring instrument is identified and verified (calibrated or verified). The conditions of use, handling and storage of instruments are established and adequate.
How can we reduce the costs of calibrating certain equipment and at the same time reward the best suggestions from staff?
Simple solution: do not calibrate multimeters and other small instruments every year but systematically buy new ones. Each instrument purchased usually has at least one year of warranty (during which neither calibration nor verification is required). Purchasing these instruments costs much less than calibration (by an external company). Old instruments with an expired warranty period (but almost always in perfect condition) are removed from the inventory and then distributed by top management to people who have distinguished themselves recently.
Each user is trained. Records of calibration and verification results are retained.
When equipment no longer complies, specific actions are taken on both the equipment and the affected productany outcome of a process or activity (see also ISO 9000, 3.4.2).
.jpg) Minute of relaxation. Cf. game: Supplier
Minute of relaxation. Cf. game: Supplier
4.4 Improvement
(Requirements 292 to 309)
Management review, inputs, outputs, conduct an audit, continual improvement

No system is perfect
Top management plans and carries out a QMSquality management system review (at least once a year). The conclusions of the review are recorded (see figure 4-8).

Its purpose is to review whether the quality management systemset of processes allowing the achievement of the quality objectives (see also ISO 9000, 3.2.3) is:
- relevant
- effective and
- appropriate to the company's vision
Management review: periodic survey carried out by top management of the management system for its continual improvement
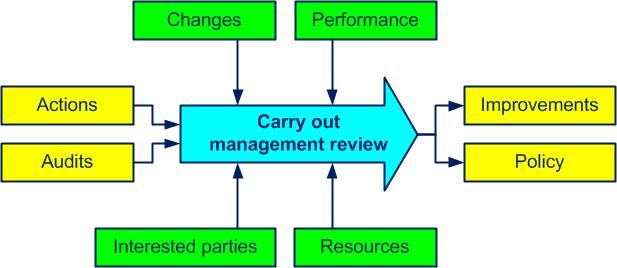
Figure 4-8. The Carry out management review process
Inputs of the review include:
- audit results
- inspection results
- the situation of the necessary resources
- the results of the assessment of compliance with legal and regulatory requirements
- feedback from interested parties (satisfaction and complaints)
- the level of achievement of quality objectives and associated indicators
- monitoring of actions:
- resulting from decisions from the previous management review
- handling nonconformities
- information on the performance of:
- processes, products and services
- competitors
- external providers
- partners
- changes in external and internal issues that may affect the quality management system and the associated threats and opportunities
- inventory of actions implemented in response to improvement risks
Review outputs include decisions related to:
- opportunities for improvement of the:
- quality management system and its processes
- product and service
- needs for modification of the quality management system (quality policy, quality objectives)
- resource needs for:
- maintaining the quality management system and continually improve its effectiveness
- increase the satisfaction of customers and other interested parties by respecting their requirements
If you can’t measure it, you can’t manage it. Peter Drucker
Processes of:
- monitoring
- measuring
- analysis and
- continual improvement
are planned and implemented to:
- demonstrate product and process conformity
- ensure product and process conformity
- ensure conformity of the QMS
- improve the effectiveness of the QMS
Measures are applied (including statistical techniques) to measure the performance of:
- customer satisfaction
- QMS conformity (internal audits)
- process control
- product control
Performance: measurable and expected results of the management system
Monitoring customeranyone who receives a product (see also ISO 9000, 3.3.5) perception of their level of satisfaction is an essential indicator of QMSquality management system performance.
After a signal from a group manager, a financial analysis of the activities of the quality department of our site was carried out. Receiving inspections were particularly targeted. To everyone's surprise it turned out that the cost was really disproportionate to that of the nonconformities found.
A reduction in activities (and a transfer of staff) was quickly implemented.
The internal audit (see figure 4-9) makes it possible to assess the conformity and effectiveness of the QMSquality management system and helps to improve its effectiveness.

Figure 4-9. The Conduct au audit process
Conformity is determined in relation to:
- the planned arrangements for the production of the product
- meeting the company's QMS requirements
- compliance with legal and regulatory requirements
Audit: systematic and independent survey to determine whether activities and results comply with pre-established measures and are capable of achieving the objectives
An audit is of:
- the QMS
- a process
- a product
Audit evidence: demonstrably true data related to audit criteria
Examples of audit evidence:
- process sheet
- job description
- training attendance sheet
- information on customer returns
- level of indicators
Audit planning (annual audit program) is done based on the state and importance of processes and products, without forgetting the results of previous audits. An auditor cannot audit their own department as:
No one should be a judge in his own case. Latin proverb
The audit follow-up (which can be a complementary audit) makes it possible to verify the implementation of the identified improvement opportunities. The results of internal audits are one of the inputs of the management review and make it possible to find areas for improvement of the QMSquality management system.
 Minute of relaxation. Cf. the “The quality engineer and the shepherd” joke.
Minute of relaxation. Cf. the “The quality engineer and the shepherd” joke.
Internal audit activities are carried out using the ISO 19 011 standard as a basis (see T 35v15 Internal audit ISO 9001).
A production manager thought that delivering on time at all costs is a top priority.
He had to deliver parts for an automotive customer. Having not received solder paste within the planned time frame, he gave the order to use expired solder paste. The delivery was made on time. The customer, after a few tests, returned the entire batch as non-compliant. The financial penalty was enormous. This was, a few weeks later, one of the causes of the liquidation of the company.
The production director hid his waiver decision from the customer and his quality manager. Expired solder paste should be destroyed as soon as the expiration date has passed. Two fatal malfunctions.
Qualities shine from afar, defects from up close. Victor Hugo
Continual improvement: process allowing the improvement of the global performance of the organization
To improve the efficiency of the QMSquality management system, opportunities for improvement must be identified in areas such as:
- customer satisfaction
- customer complaint rates
- working conditions
- on-time delivery
- staff skills
- cost reduction
- net margin
The continual improvementpermanent process allowing the improvement of the global performance of the organization (see also ISO 9000, 3.2.13 and ISO 14 001, 3.2) processactivities which transform inputs into outputs (see also ISO 9000, 3.4.1) is based, among other things, on:
- audit results
- necessary resources
- data analysis
- staff suggestions
- setting new improvement targets
- finding and justifying solutions
- implementing solutions and measuring results
- formalizing changes when objectives are reached
- regular updating of quality plans
- for inspection activities, all acceptance criteria are defined
- missing acceptance criteria for certain products
- certain legal and regulatory requirements are not taken into account
More examples of good practices and bad practices can be found in annexes 07 and 08.
To find out more about QMS requirements, go to module T 15v15 "ISO 9001 readiness version 2015".
.jpg) Minute of relaxation. Cf. game: Effectiveness
Minute of relaxation. Cf. game: Effectiveness
The rest of the T 49 Lean approach training is accessible on this page.
